The Directorate of Technological Development (VDTEC) operates with four programs involving Bacterial Vaccines, Viral Vaccines, Reactives for Diagnosis and Biopharmaceuticals, aiming at the development of products for the prevention, diagnosis and treatment of diseases of great impact, or rare diseases, relevant for public health. Such programs are supported by seven laboratories and three centers, organized by technological platforms and sized to meet the demands of all projects for development and/or technology transfer of products and processes at the Unit, as well as for incremental innovation of registered products, when required.
VDTEC's laboratories and centers operate in accordance with national and international standards, comprising an area of 2,292.75 m2, organized into 10 areas of knowledge: Immunology (Laboratory of Immunological Technology - LATIM), Molecular Biology (Recombinant Technology - LATER) , Macromolecule Biochemistry (Laboratory of Macromolecule Chemistry- LAMAM), Virology (LATEV), Bacteriology (LATEB), Reactives for Diagnosis (LATED), Monoclonal Antibodies (LATAM), Formulation and Lyophilization (NULEX), Biosafety (NBIOS), as well as a washing and sterilization area (NULME). VDTEC also has an administrative support center (AADM-VDTEC) and a pilot plant, the first in the country, with characteristics of Good Manufacturing Practices (GMP) and designed for the production of pilot batches of biological products for clinical studies.
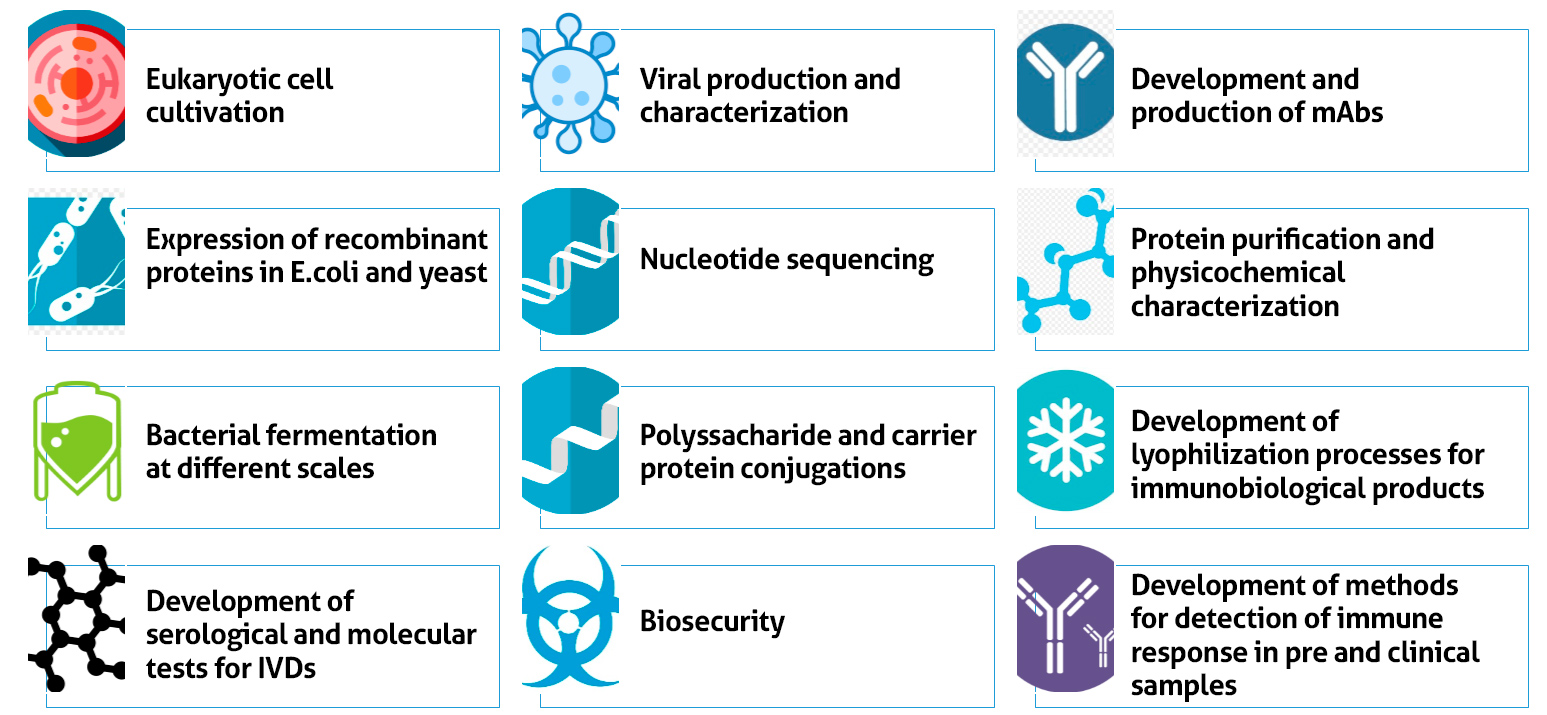
The strategy adopted by VDTEC is based on the execution of projects on different technological platforms (Fig.1), where facilities and equipment are shared according to the need, stage and priorities of the projects, optimizing the efficient use of the existing infrastructure. These technological platforms include expression of recombinant antigens, protein purification and physicochemical characterization, cultivation of eukaryotic and prokaryotic cells, development and production of mabs, nucleotide sequencing, viral production and characterization, bacterial fermentation at different scales, conjugations of active molecules, serological and molecular tests, methods for detecting immune response and lyophilization and formulation processes. Additionally, VDTEC has the support of the Biosafety Center, generating compliance of its processes with CTNBio. These platforms are also available to provide external services, contributing to advances in the biotechnology area.
Autochthonous development projects have played an important role in the registration and supply of new products, as well as in partnerships with industries and universities, contributing to the formation of relevant skills in the Institution. Partnerships with organizations outside Fiocruz, represented by joint development agreements, have added strategic knowledge in several areas of knowledge such as: project management, process development, epidemiological studies, clinical studies, among others.
We have competence in all phases of biological product development, from pre-development (product definition and proof of principle confirmation), experimental development (bench scale prototype stages), pre-clinical studies, pilot batch procurement (experimental development of the prototype on a pilot scale for clinical trials) to the elaboration and execution of clinical protocols (clinical evaluation of the efficacy and safety of the use of the product in humans).
The continuous investment in the innovation chain and in the modernization of physical areas, acquisition and maintenance of equipment, as well as the mastery of cutting-edge technologies and partnerships with other institutions - public and private - contribute to the recognized level of excellence of our technological development.


